2na S 2h2o L 2naoh Aq H2 G
Cambio de energía de gibbs. Assume that delta H.

Which Is The Oxidising Agent In The Following Equation Haso2 Aq Sn 2 Aq H Aq As S Sn 4 Aq H2o I
Fe CuSO4 FeSO4 Cu B.

. Question2Nas 2H2Ol 2NaOHaq H2gFrom the equation above calculate the mass of sodium hydroxide produced by 23g of sodiumOptionsA 040gB Toggle navigation. Fe CuSO4 FeSO4 Cu. Ca 2HCl CaCl2 H2.
20 PbSO4s 2 H2Ol充电时阳极PbSO4s 2 H2Ol -2e PbO2s 4H aq SO42- aq 阴极PbSO4s 2e Pbs SO42- aq 9氢氧燃料电池P77总反应2H2 O2 2H2O酸性条件负极 2H2 -4e- 4H 正极O2 4H 4e- 2 H2碱性条件负极2H2 4OH -4e- 4 H2O 正极O2 2H2O 4e- 4OH10. Single Replacement or substitution Reaction. In each of the following reactions decide which reactant is oxidized and which is reduced.
For the reaction 2Na s 2H2O l ----- 2NaOH aq H2 g delta H -3686 kJ and delta S -153 JK The maximum amount of work that could be done when 247 moles of Na s react at 295 K 1 atm is______. So it won electrons. Option C Explanation 2Na s 2H 2 O l 2NaOH aq H 2 g Relative molecular mass of NaOH 23 16 1 40smol -1 2 23g of sodium react with water to produce 2 40g of NaOH 23g of sodium will react with water to produce 23 40 2 23 2 400g Previous Next Go back to Chem classroom.
This problem has been solved. 2Nas2H2Ol2NaOHaqH2g 115g of sodium is allowed to react completely with waterthe resulting solution is diluted to 250cm3calculate the concentration in mol dm3 of the resulting hydroxide solution. Ca 2HCl CaCl2 H2.
It has released and electron. What mass of phosphorus will be needed to produce 325. Jul 31 2013 2 Zuhsid.
H from water has gained an electron so it changed the oxidation state from 1 to 0. 2Na s2H₂O l2NaOH aqH₂ g Sodium changes from 0 oxidation state to 1. 2Nas 2H2Ol---- 2NaOHaq H2g 403 grams.
Designate the oxidizing agent and reducing agent. As the oxidation state has decreased the element was reduced. Đề thi minh họa kỳ thi THPT Quốc Gia môn Hóa Học năm 2019.
Câu hỏi trong đề. Cu H2SO4 CuSO4 H2. Phosphorous burns in air to produce a phosphorus oxide in the following reaction.
2Nas 2H2Ol 2NaOHaq H2g In this chemical equation H2O is the oxidizing agent. 2Nas 2H2Ol---- 2NaOHaq H2g 184 grams. Up to 256 cash back Get the detailed answer.
Na 2 O es H 2 O r NaOH es ΔG 0 298 -148 kj. It has been oxidized. A 2 Mgs O2g 2 MgOs.
What type of reaction is. 2Na s 2H 2 O l 2NaOH aq H 2 g Double Replacement or metathesis Reaction. A CdCl2aq Na2Saq CdSs 2 NaClaq b 2 Cas O2g 2 CaOs c CaOH2s 2 HClaq CaCl2aq 2 H2Ol 58.
Messages 234 Reaction score 90 Points 38. 2Na 2H2O 2NaOH H2 D. Phương trình hóa học nào sau đây là sai.
How can we tell that it has reduced if. Science Chemistry QA Library 2Na s 2H2O 2NaOH aq H2 g Multiple Choice O O O single-displacement double-displacement combination decomposition combustion K. A redox reaction.
Na 2 O H 2 O 2NaOH Verificar el equilibrio La reacción de la interacción de óxido de sodio y agua con la formación de hidróxido de sodio. How many grams of sodium will react with water to produce 40 mol of hydrogen in the following reaction. Cu H2SO4 CuSO4 H2 C.
2Na s 2H 2 O l 2NaOH aq H 2g Relative molecular mass of NaOH 23 16 1 40smol-1 2 23g of sodium react with water to produce. 2Na 2H2O 2NaOH H2.

Use Oxidation States To Identify The Oxidizing Agent And The Reducing Agent In The Following Redox Reaction 2na S 2h2o L 2naoh Aq H2 G Reducing Agent Oxidizing Agent Homework Study Com

Solved Consider The Reaction 2na S 2h2o L 2naoh Aq Chegg Com
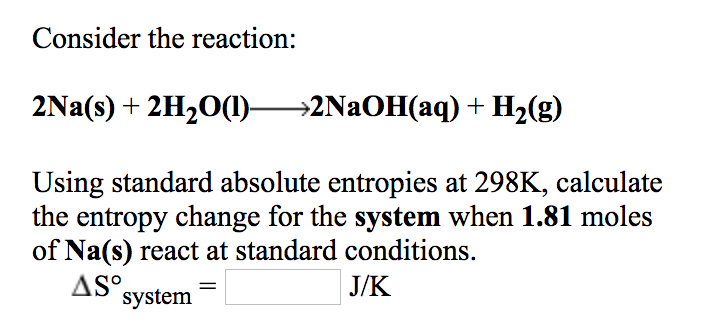
Solved Consider The Reaction 2na S 2h2o 1 2naoh Aq Chegg Com
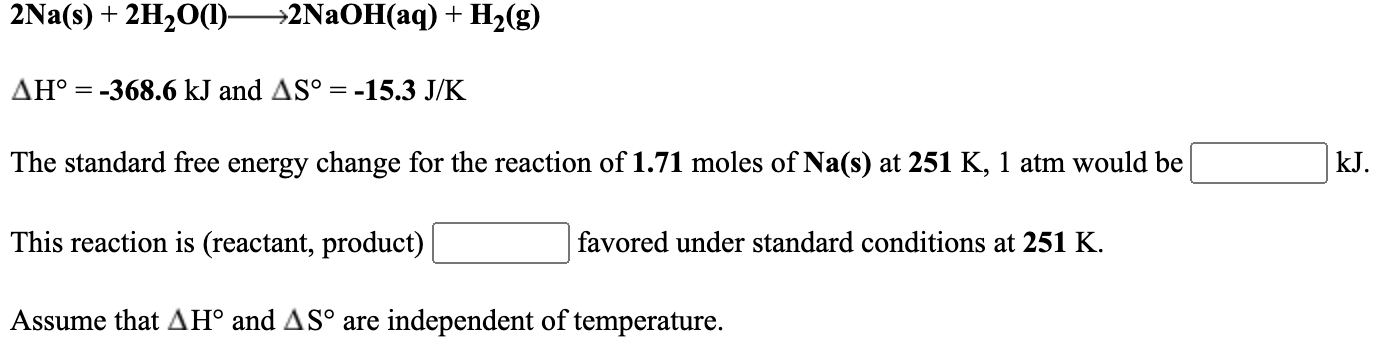
No comments for "2na S 2h2o L 2naoh Aq H2 G"
Post a Comment